화이자(Pfizer) 백신 개발 성공...연말까지 최대 2천만명 제공 가능
화이자(Pfizer) 백신 개발 성공...연말까지 최대 2천만명 제공 가능
제약회사 화이자(Pfizer)가 백신 개발에 성공했다.
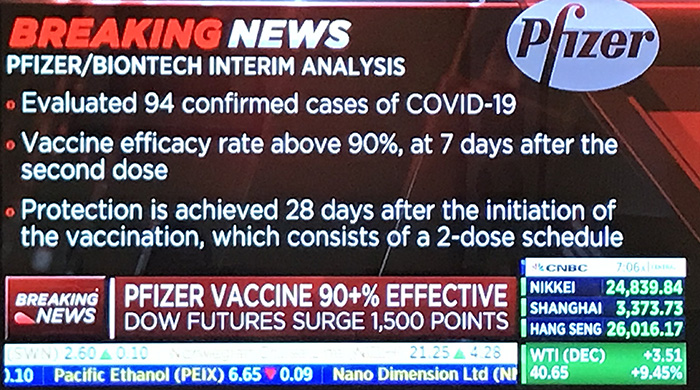
화이자의 바이노테크(BioNTech)는 최근 제 3차 시험에서 코로나19 환자 94건에 대해 90% 이상의 예방 효능이 있었다고 밝혔다.
94건의 사례에서 총 4만3천538명의 참가자(42% 다양한 배경)에 심각한 부작용은 발견되지 않았다.
미 식품의약국(FDA)에 긴급 승인을 거쳐 연말까지 1천500만-2천만명에게 제공할 수 있다.
작가 월터 아이작슨도 임상 실험에 참가했다.
PFIZER AND BIONTECH ANNOUNCE VACCINE CANDIDATE AGAINST COVID-19 ACHIEVED SUCCESS IN FIRST INTERIM ANALYSIS FROM PHASE 3 STUDY
Monday, November 09, 2020 - 06:45am
Vaccine candidate was found to be more than 90% effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection in the first interim efficacy analysis
Analysis evaluated 94 confirmed cases of COVID-19 in trial participants
Study enrolled 43,538 participants, with 42% having diverse backgrounds, and no serious safety concerns have been observed; Safety and additional efficacy data continue to be collected
Submission for Emergency Use Authorization (EUA) to the U.S. Food and Drug Administration (FDA) planned for soon after the required safety milestone is achieved, which is currently expected to occur in the third week of November
Clinical trial to continue through to final analysis at 164 confirmed cases in order to collect further data and characterize the vaccine candidate’s performance against other study endpoints
NEW YORK & MAINZ, GERMANY--(BUSINESS WIRE)-- Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced their mRNA-based vaccine candidate, BNT162b2, against SARS-CoV-2 has demonstrated evidence of efficacy against COVID-19 in participants without prior evidence of SARS-CoV-2 infection, based on the first interim efficacy analysis conducted on November 8, 2020 by an external, independent Data Monitoring Committee (DMC) from the Phase 3 clinical study.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201109005539/en/
“Today is a great day for science and humanity. The first set of results from our Phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine’s ability to prevent COVID-19,” said Dr. Albert Bourla, Pfizer Chairman and CEO. “We are reaching this critical milestone in our vaccine development program at a time when the world needs it most with infection rates setting new records, hospitals nearing over-capacity and economies struggling to reopen. With today’s news, we are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis. We look forward to sharing additional efficacy and safety data generated from thousands of participants in the coming weeks.”After discussion with the FDA, the companies recently elected to drop the 32-case interim analysis and conduct the first interim analysis at a minimum of 62 cases. Upon the conclusion of those discussions, the evaluable case count reached 94 and the DMC performed its first analysis on all cases. The case split between vaccinated individuals and those who received the placebo indicates a vaccine efficacy rate above 90%, at 7 days after the second dose. This means that protection is achieved 28 days after the initiation of the vaccination, which consists of a 2-dose schedule. As the study continues, the final vaccine efficacy percentage may vary. The DMC has not reported any serious safety concerns and recommends that the study continue to collect additional safety and efficacy data as planned. The data will be discussed with regulatory authorities worldwide.
“I want to thank the thousands of people who volunteered to participate in the clinical trial, our academic collaborators and investigators at the study sites, and our colleagues and collaborators around the world who are dedicating their time to this crucial endeavor,” added Bourla. “We could not have come this far without the tremendous commitment of everyone involved.”
“The first interim analysis of our global Phase 3 study provides evidence that a vaccine may effectively prevent COVID-19. This is a victory for innovation, science and a global collaborative effort,” said Prof. Ugur Sahin, BioNTech co-founder and CEO. “When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality. We will continue to collect further data as the trial continues to enroll for a final analysis planned when a total of 164 confirmed COVID-19 cases have accrued. I would like to thank everyone who has contributed to make this important achievement possible.”
The Phase 3 clinical trial of BNT162b2 began on July 27 and has enrolled 43,538 participants to date, 38,955 of whom have received a second dose of the vaccine candidate as of November 8, 2020. Approximately 42% of global participants and 30% of U.S. participants have racially and ethnically diverse backgrounds. The trial is continuing to enroll and is expected to continue through the final analysis when a total of 164 confirmed COVID-19 cases have accrued. The study also will evaluate the potential for the vaccine candidate to provide protection against COVID-19 in those who have had prior exposure to SARS-CoV-2, as well as vaccine prevention against severe COVID-19 disease. In addition to the primary efficacy endpoints evaluating confirmed COVID-19 cases accruing from 7 days after the second dose, the final analysis now will include, with the approval of the FDA, new secondary endpoints evaluating efficacy based on cases accruing 14 days after the second dose as well. The companies believe that the addition of these secondary endpoints will help align data across all COVID-19 vaccine studies and allow for cross-trial learnings and comparisons between these novel vaccine platforms. The companies have posted an updated version of the study protocol at https://www.pfizer.com/science/coronavirus.
Pfizer and BioNTech are continuing to accumulate safety data and currently estimate that a median of two months of safety data following the second (and final) dose of the vaccine candidate – the amount of safety data specified by the FDA in its guidance for potential Emergency Use Authorization – will be available by the third week of November. Additionally, participants will continue to be monitored for long-term protection and safety for an additional two years after their second dose.
Along with the efficacy data generated from the clinical trial, Pfizer and BioNTech are working to prepare the necessary safety and manufacturing data to submit to the FDA to demonstrate the safety and quality of the vaccine product produced.
Based on current projections we expect to produce globally up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021.
Pfizer and BioNTech plan to submit data from the full Phase 3 trial for scientific peer-review publication.
About Pfizer: Breakthroughs That Change Patients’ Lives
At Pfizer, we apply science and our global resources to bring therapies to people that extend and significantly improve their lives. We strive to set the standard for quality, safety and value in the discovery, development and manufacture of health care products, including innovative medicines and vaccines. Every day, Pfizer colleagues work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. Consistent with our responsibility as one of the world's premier innovative biopharmaceutical companies, we collaborate with health care providers, governments and local communities to support and expand access to reliable, affordable health care around the world. For more than 150 years, we have worked to make a difference for all who rely on us. We routinely post information that may be important to investors on our website at www.Pfizer.com. In addition, to learn more, please visit us on www.Pfizer.com and follow us on Twitter at @Pfizer and @Pfizer News, LinkedIn, YouTube and like us on Facebook at Facebook.com/Pfizer.
Pfizer Stuns Experts With Early Data that Vaccine Is More Than 90% Effective -NYT-
Pfizer announced positive early results from its coronavirus vaccine trial, cementing the lead in a frenzied global race that has unfolded at record-breaking speed.
https://www.nytimes.com/2020/11/09/health/covid-vaccine-pfizer.html






-Elaine-